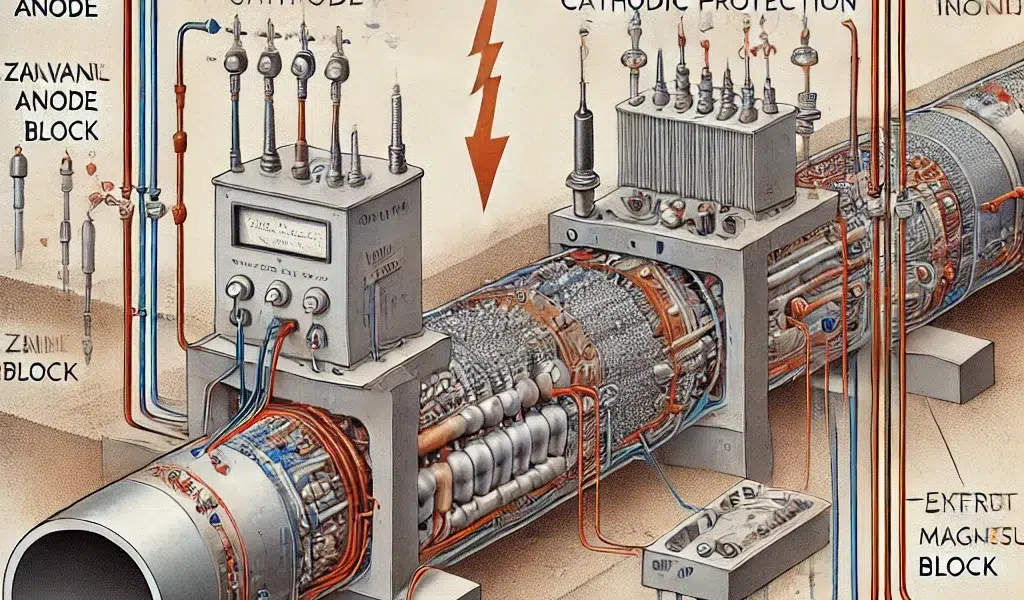
Corrosion is a natural but damaging process that can significantly impact the lifespan and integrity of metal structures. From pipelines to bridges, and ships to underground storage tanks, corrosion can lead to catastrophic failures, causing environmental damage, economic losses, and even threats to human safety. One of the most effective methods to prevent corrosion is Cathodic Protection (CP). In this detailed technical blog, we will explore the principles, types, applications, and benefits of cathodic protection, along with a thorough discussion on how it works and its importance in various industries.
Understanding Corrosion
Before delving into cathodic protection, it’s essential to understand the phenomenon it is designed to combat—corrosion. Corrosion is the gradual destruction of metals due to chemical reactions with their environment. The most common type of corrosion is electrochemical, where metal atoms lose electrons and become oxidized, leading to the formation of rust in the presence of oxygen and moisture. This process is akin to the metal reverting to its more stable oxide form, as found in nature.
Corrosion can manifest in various forms, including uniform corrosion, pitting, galvanic corrosion, crevice corrosion, and stress corrosion cracking. The specific type of corrosion depends on the metal’s composition, its environment, and mechanical stresses it experiences. Regardless of the type, corrosion poses a significant challenge to the longevity and functionality of metal structures.
What is Cathodic Protection?
Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. In simpler terms, cathodic protection involves the application of a direct electrical current or the use of a sacrificial anode to counteract the corrosion process. This method effectively suppresses the electrochemical reactions that cause metal deterioration, thereby extending the lifespan of the protected structure.
The fundamental principle behind cathodic protection is based on electrochemistry. In an unprotected metal structure, the surface is composed of both anodic (positively charged) and cathodic (negatively charged) areas. The anodic areas are where oxidation (corrosion) occurs, while the cathodic areas remain relatively intact. By applying cathodic protection, the entire metal surface is converted into a cathodic region, effectively halting the corrosion process.
Types of Cathodic Protection
There are two primary types of cathodic protection: Galvanic (Sacrificial Anode) Cathodic Protection and Impressed Current Cathodic Protection (ICCP). Both methods serve the same purpose but operate differently and are suited for different applications.
1. Galvanic (Sacrificial Anode) Cathodic Protection

In galvanic cathodic protection, a more active (less noble) metal, known as the sacrificial anode, is connected to the structure that needs protection. This sacrificial anode is chosen because it has a higher tendency to corrode (oxidize) than the protected metal. When the sacrificial anode corrodes, it releases electrons that flow to the protected metal, thereby reducing the metal’s potential and preventing its corrosion.
Common materials used for sacrificial anodes include zinc, magnesium, and aluminum alloys. These materials are selected based on the environment in which they will be used and the metal they are protecting. For example, zinc is commonly used in seawater applications, while magnesium is preferred for underground pipelines and tanks.
Advantages:
- Simple installation and maintenance.
- No external power source required.
- Suitable for small to medium-sized structures.
Disadvantages:
- Limited lifespan of sacrificial anodes, requiring periodic replacement.
- Ineffective for large structures or in environments with high resistivity.
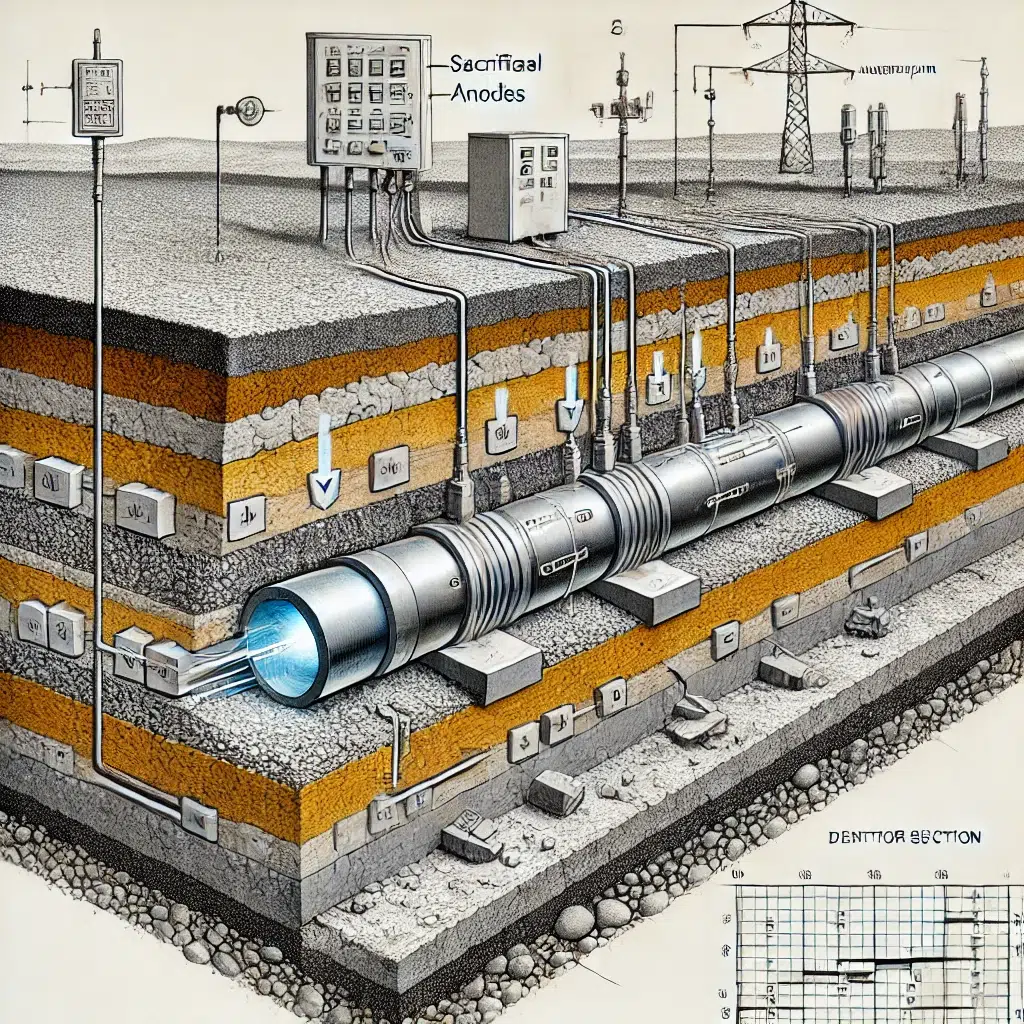
2. Impressed Current Cathodic Protection (ICCP)
Impressed current cathodic protection is a more sophisticated method that uses an external power source to provide a continuous flow of direct current (DC) to the metal structure. Unlike galvanic CP, where the sacrificial anode is consumed over time, ICCP uses inert anodes (typically made of materials like titanium, mixed metal oxides, or graphite) that do not corrode easily.
In an ICCP system, a rectifier converts alternating current (AC) from the power supply to direct current, which is then applied to the metal structure. The inert anodes are connected to the positive terminal of the rectifier, while the protected metal is connected to the negative terminal. This setup ensures that the entire metal surface remains cathodic, effectively preventing corrosion.
Advantages:
- Long-term protection with minimal maintenance.
- Suitable for large and complex structures.
- Can be adjusted to varying environmental conditions.
Disadvantages:
- Higher initial cost and complexity.
- Requires a reliable power source.
- Potential for overprotection, leading to hydrogen embrittlement.
Applications of Cathodic Protection
Cathodic protection is widely used in various industries to protect metal structures from corrosion. Some of the most common applications include:
1. Underground Pipelines
Underground pipelines, especially those used for transporting oil, gas, and water, are highly susceptible to corrosion due to their exposure to soil, moisture, and varying environmental conditions. Cathodic protection is extensively used to safeguard these pipelines, ensuring their integrity and preventing leaks or ruptures that could have catastrophic consequences.
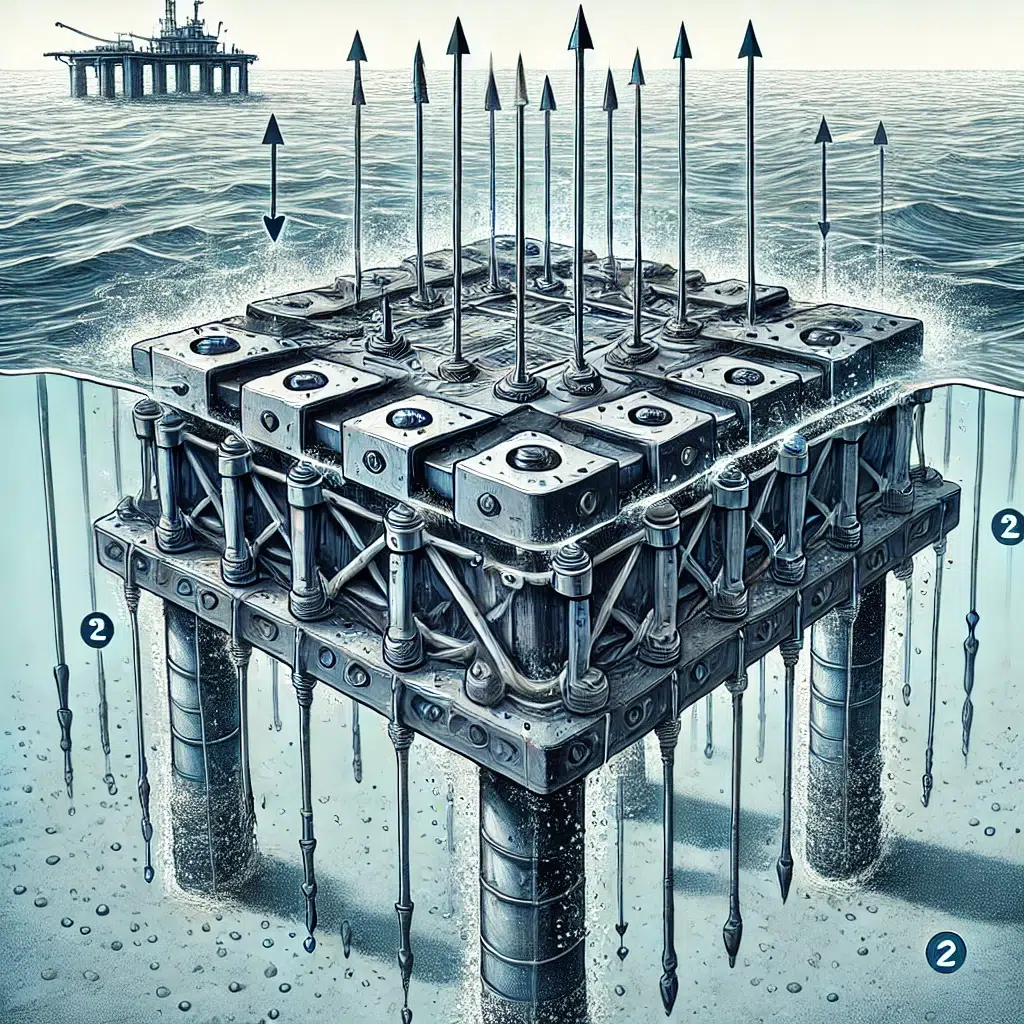
2. Marine Structures
Marine environments are highly corrosive due to the presence of saltwater, which accelerates the corrosion process. Cathodic protection is crucial for protecting ships, offshore platforms, docks, and other marine structures from the harsh effects of seawater. Both galvanic and impressed current systems are employed depending on the size and complexity of the structure.
3. Storage Tanks
Aboveground and underground storage tanks, particularly those containing hazardous materials, are at risk of corrosion, which can lead to leaks and environmental contamination. Cathodic protection is used to protect the exterior and interior surfaces of these tanks, ensuring their long-term durability and preventing costly repairs.
4. Bridges
Bridges, especially those made of steel, are exposed to various environmental factors such as moisture, de-icing salts, and fluctuating temperatures, all of which contribute to corrosion. Cathodic protection is applied to critical areas of bridges, such as support beams and pylons, to prevent corrosion-related failures and extend the lifespan of the structure.
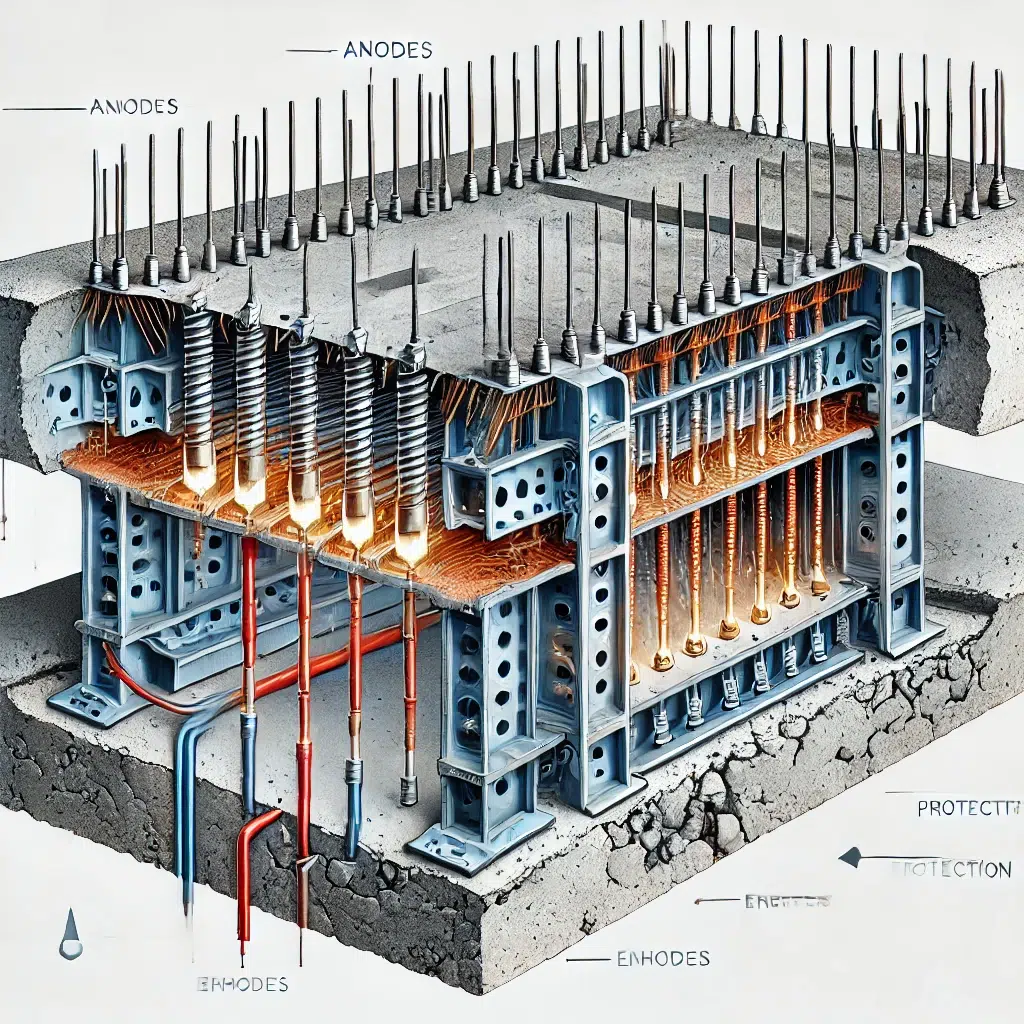
5. Reinforced Concrete Structures
Reinforced concrete structures, such as parking garages, highways, and buildings, contain steel reinforcement bars (rebar) that are vulnerable to corrosion when exposed to moisture and chlorides. Cathodic protection is used to protect the rebar from corrosion, ensuring the structural integrity of the concrete over time.
Designing a Cathodic Protection System
The design of a cathodic protection system is a critical step in ensuring its effectiveness. Several factors must be considered during the design process, including:
- Material Properties: The type of metal being protected and its electrochemical properties influence the choice of cathodic protection method (galvanic or ICCP) and the type of anode material used.
- Environmental Conditions: The environment in which the structure is located, such as soil resistivity, water salinity, and temperature, affects the design of the CP system. For example, high-resistivity soils may require impressed current systems due to the limited effectiveness of galvanic anodes.
- Structure Size and Complexity: Larger and more complex structures, such as long pipelines or offshore platforms, may require multiple anode locations or a combination of galvanic and impressed current systems to ensure uniform protection.
- Current Density Requirements: The current density required to protect the metal surface from corrosion is a key parameter in the design of a cathodic protection system. It depends on factors such as the type of metal, the corrosive environment, and the desired level of protection.
- Monitoring and Maintenance: Cathodic protection systems require regular monitoring and maintenance to ensure they continue to provide adequate protection. Monitoring techniques include potential measurements, current output checks, and anode inspections. Maintenance activities may involve adjusting rectifier settings, replacing sacrificial anodes, or repairing damaged components.
Benefits of Cathodic Protection
Cathodic protection offers numerous benefits, making it an essential tool in the fight against corrosion. Some of the key benefits include:
- Extended Lifespan of Structures: By preventing or significantly reducing corrosion, cathodic protection extends the lifespan of metal structures, reducing the need for costly repairs or replacements.
- Cost Savings: While the initial installation of a cathodic protection system may be expensive, the long-term cost savings from reduced maintenance, repairs, and replacements far outweigh the initial investment.
- Environmental Protection: Cathodic protection helps prevent leaks and spills from pipelines, storage tanks, and other structures that could cause environmental contamination. This is particularly important in industries such as oil and gas, where the consequences of corrosion-related failures can be severe.
- Safety: Corrosion-related failures can lead to accidents, injuries, and loss of life. By protecting critical infrastructure from corrosion, cathodic protection enhances safety and reduces the risk of catastrophic events.
- Compliance with Regulations: Many industries are subject to strict regulations regarding the maintenance and integrity of metal structures. Cathodic protection helps companies comply with these regulations, avoiding fines, penalties, and legal liabilities.
Challenges and Limitations of Cathodic Protection
While cathodic protection is highly effective, it is not without its challenges and limitations. Some of the common challenges include:
- Overprotection: In impressed current systems, excessive current can lead to overprotection, causing hydrogen embrittlement in certain metals, such as high-strength steels. This can weaken the metal and increase the risk of failure.
- Power Supply Dependence: Impressed current systems rely on a continuous power supply, making them vulnerable to power outages or failures. Backup power systems or redundant designs may be necessary to ensure uninterrupted protection.
- Complexity and Cost: The design, installation, and maintenance of cathodic protection systems can be complex and costly, particularly for large or remote structures. However, these costs are often justified by the long-term benefits of corrosion prevention.
- Anode Depletion: In galvanic systems, sacrificial anodes are consumed over time and must be replaced periodically. Failure to replace depleted anodes can result in a loss of protection and increased risk of corrosion.
- Interference Issues: Cathodic protection systems can sometimes interfere with other nearby structures, particularly in densely populated or industrial areas. Proper design and careful monitoring are required to minimize such interference.
Conclusion
Cathodic protection is a proven and effective method for combating corrosion in a wide range of metal structures. Whether using galvanic anodes or impressed current systems, cathodic protection offers significant benefits in terms of extending the lifespan of structures, reducing maintenance costs, and ensuring safety and environmental protection. However, the successful implementation of cathodic protection requires careful design, regular monitoring, and ongoing maintenance.
As industries continue to rely on metal structures for critical infrastructure, the importance of cathodic protection cannot be overstated. By understanding the principles, applications, and challenges of cathodic protection, engineers and asset managers can make informed decisions that enhance the durability and reliability of their structures, ensuring they remain safe and functional for years to come.